In medical practice, terms like “fraboc” (often written as FRA-BOC) occasionally appear, sparking confusion and curiosity among patients, researchers, and even healthcare professionals. Although not commonly discussed in contemporary practice, the concept behind fraboc remains significant in the context of cancer risk assessment. The term refers to a structured framework used to assess the inherited risk of breast and ovarian cancer based on family history. Despite its decline in use as a standalone tool, fraboc laid the foundation for many modern approaches to understanding genetic risk. This article will explore the essence of fraboc, its applications, limitations, and its continuing relevance in contemporary medical practice.
What is Fraboc?
Fraboc is not a disease, diagnosis, or treatment. Rather, it refers to a decision-support framework designed to help clinicians assess the inherited risk of cancer, particularly breast and ovarian cancer, based on a patient’s family history. This framework is built on the idea of recognizing patterns in family medical history, with a focus on identifying those at an increased risk due to inherited genetic factors.
The fraboc system doesn’t predict cancer development or serve as an absolute measure of risk. Instead, it helps healthcare professionals categorize patients into different risk levels based on family history. It allows clinicians to make more informed decisions about the need for follow-up actions, genetic testing, or referrals to specialists.
The Core Concept: Family History and Patterns
At its core, fraboc emphasizes pattern recognition across generations. Rather than focusing solely on an individual’s cancer diagnosis, it looks at the occurrence of cancer in multiple generations within a family. If certain cancers, like breast or ovarian cancer, appear repeatedly in younger family members or in specific combinations, this could indicate an inherited predisposition that warrants further attention.
Fraboc was created to make sense of complex family history information and organize it into categories of risk. It is not a diagnostic tool but a means of evaluating the likelihood that a hereditary cancer syndrome is present, based on the family’s medical history. This helps guide clinicians to determine the appropriate next steps for monitoring and potential preventive measures.
The Role of Family History in Cancer Risk
Fraboc relies heavily on family history as the primary tool for assessing risk. By collecting detailed information about the cancers in a patient’s family, particularly focusing on the ages of diagnosis and the relationships between affected individuals, fraboc aims to identify individuals who may be at a higher genetic risk. This information typically spans three generations if possible, providing a broad perspective of the family’s medical history.
The idea behind using family history is based on the understanding that some types of cancer are more likely to occur in individuals who have a genetic predisposition passed down through generations. For example, the presence of multiple cases of breast cancer in close relatives at young ages may indicate a hereditary form of the disease, such as those associated with mutations in the BRCA1 or BRCA2 genes.
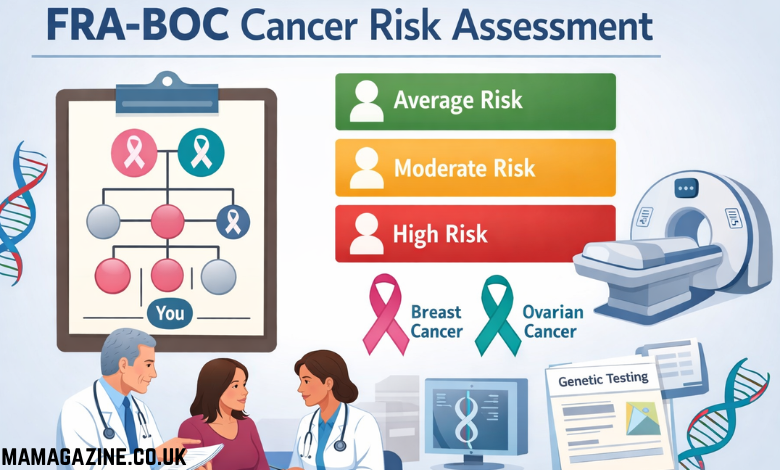
The Risk Categories: From Average to High Risk
One of the strengths of the fraboc framework is that it does not rely on rigid categories like “high risk” or “no risk.” Instead, it places individuals into broad categories that reflect varying levels of risk. These categories typically include:
- Average Risk: Individuals who have a family history of cancer but do not show any significant patterns that suggest an inherited risk.
- Moderately Increased Risk: Individuals who have one or more family members diagnosed with cancer at a relatively young age or in multiple family members, suggesting a slightly higher genetic risk.
- Significantly Increased Risk: Individuals who show strong patterns of cancer occurring at young ages across multiple generations, indicating a potentially high genetic risk.
The goal of this categorization is to help healthcare professionals make decisions about the appropriate level of follow-up care, such as more frequent screenings, genetic testing, or preventive measures.
Why Fraboc Was Important
Fraboc served as an important decision-support tool in the era before more advanced genetic testing became commonplace. It was used to systematically assess family histories and reduce the potential for human error or inconsistency when making risk evaluations. This helped ensure that individuals with significant risk factors were not overlooked, allowing for early detection and preventive actions when necessary.
By taking a structured approach, fraboc provided clinicians with a standardized method for evaluating family history and assigning risk levels. This consistency was particularly important in the clinical setting, where different healthcare providers might interpret family history in different ways.
The Limitations of Fraboc
Despite its advantages, fraboc had several limitations that became evident as medical knowledge and technology advanced. Some of the key limitations include:
- Reliance on Family History Alone: Fraboc relied primarily on family history, which could be incomplete or inaccurate. Some families might not have comprehensive health records, and certain cancers might not be well documented, leading to an incomplete picture of the inherited risk.
- Lack of Genetic Information: Fraboc did not incorporate genetic testing, which has since become a more accurate way to assess inherited cancer risk. As a result, it could not identify genetic mutations like BRCA1 and BRCA2 that are associated with high risks for breast and ovarian cancer.
- No Individualized Risk Assessment: While fraboc categorized individuals based on family history, it did not account for personal risk factors, such as lifestyle choices or environmental exposures, which can also influence cancer risk.
- A Lack of Precision: The broad risk categories (average, moderately increased, and significantly increased) could sometimes be too vague, leading to uncertainty in the clinical decision-making process.
The Evolution of Risk Assessment Frameworks
Over time, frameworks like fraboc were refined and replaced by more modern approaches to cancer risk assessment. Advances in genetic research and technology have provided new tools for identifying inherited risks more accurately. Today, genetic testing plays a critical role in assessing cancer risk, allowing healthcare providers to identify mutations that may predispose individuals to specific cancers.
For example, modern risk assessment models may incorporate genetic testing, lifestyle factors, and family history to provide a more comprehensive evaluation of an individual’s cancer risk. These models are also better equipped to guide decisions about preventive measures, such as prophylactic surgeries or chemoprevention, and to inform discussions about screening schedules and treatment options.
Despite the advancements in genetic testing and more sophisticated risk models, the core principles of fraboc remain relevant. Collecting family history, identifying patterns, and making decisions based on risk levels continue to be the foundation of modern cancer risk assessment tools.
The Relevance of Fraboc Today
While fraboc is no longer used as a standalone tool in clinical practice, the underlying concept of assessing cancer risk through structured frameworks remains highly relevant. Today, the term “fraboc” may appear in older medical documents or be referenced in discussions about family history-based cancer risk, particularly in the context of genetic counseling or risk management.
In practice, the concept of structured risk assessment based on family history is still widely used, but with more advanced tools and methodologies. Healthcare professionals continue to evaluate family history as an essential component of cancer risk assessment, often in combination with genetic testing, medical imaging, and other diagnostic tools.
For patients researching their family cancer history, the presence of the term “fraboc” in medical records is usually not a cause for alarm. It indicates that family history was considered as part of a broader risk assessment process. Patients who have questions about their own cancer risk should discuss their concerns with a healthcare provider, who can explain the risk assessment process and recommend appropriate next steps.
Fraboc: A Structured Approach to Assessing Cancer Risk
1. Introduction to Fraboc
- What Fraboc Represents: Fraboc is a structured risk-assessment framework, not a disease or treatment, designed to evaluate the inherited risk of breast and ovarian cancer based on family history.
- Origins of the Term: Often written as FRA-BOC, fraboc first appeared in medical notes, older clinical documents, and academic resources, but its concept remains relevant even though it is no longer in widespread use.
- Purpose of Fraboc: The goal was to assist clinicians in determining the appropriate follow-up care, referrals, and possible preventive measures for patients based on their family cancer history.
- Role in Cancer Risk Assessment: Fraboc focuses on identifying inherited cancer risks through patterns found in a family’s medical history, especially when cancers appear at younger ages or in specific combinations across generations.
- Current Relevance: Although newer methods have surpassed fraboc, the concept of family history-based risk assessment still forms the basis for current cancer screening tools and genetic testing protocols.
2. Understanding Family History-Based Risk Assessment
- Family History as a Tool: Fraboc primarily relied on collecting detailed family history over three generations to identify patterns of inherited cancers, particularly breast and ovarian cancers.
- Patterns of Cancer: The focus was on recognizing multiple cases of cancer within a family, especially when diagnosed at younger ages or involving specific combinations of cancers, as indicators of potential genetic risks.
- Three Generations: The family history review usually spans three generations, allowing clinicians to assess the inheritance of cancer across a broader family tree.
- Types of Cancer: Although fraboc focused on breast and ovarian cancers, the principle of identifying family patterns can be applied to other types of cancer and hereditary conditions as well.
- Challenges with Family History: The limitations of relying on family history alone, such as incomplete or inaccurate medical records, highlight the need for complementary tools like genetic testing to better understand risk.
3. The Structure of Fraboc: Risk Categories
- Risk Categorization: Fraboc divided individuals into broad risk categories to help clinicians make informed decisions about follow-up care.
- Average Risk: This category included individuals who had a family history of cancer but did not show significant patterns that suggested inherited genetic risk.
- Moderately Increased Risk: This category identified individuals with a family history showing patterns of cancer occurring in one or more relatives, typically diagnosed at younger ages.
- Significantly Increased Risk: Individuals in this category exhibited strong patterns of cancer across generations, suggesting a higher likelihood of inherited genetic mutations, such as BRCA1 and BRCA2.
- Avoiding Rigid Labels: Fraboc’s approach was not to label individuals as definitively “high risk” or “no risk” but to place them into categories to guide further action, reducing guesswork and ensuring appropriate monitoring.
4. Benefits of the Fraboc Framework
- Consistency Across Clinicians: Fraboc’s structured approach ensured that different healthcare providers, looking at similar family histories, would come to similar conclusions regarding risk levels, making it easier to decide on next steps.
- Helps Avoid Overlooking Risk: By focusing on family patterns, fraboc helped ensure that individuals with meaningful genetic risk signals were not overlooked or dismissed.
- Practical and Simple: Fraboc provided a straightforward, easy-to-understand framework for clinicians to use in a clinical setting, improving the efficiency of risk assessments.
- Limited Need for Specialized Knowledge: The use of fraboc did not require highly specialized genetic knowledge, making it accessible to general practitioners and healthcare providers in different clinical settings.
5. The Limitations of Fraboc
- Incomplete Family History: One major limitation was that fraboc relied on an accurate family history, which might be incomplete, leading to an incomplete risk assessment.
- No Genetic Testing Integration: Unlike modern risk assessment tools, fraboc did not incorporate genetic testing, missing out on detecting specific genetic mutations linked to higher cancer risks.
- Limited to Known Cancers: The framework focused primarily on breast and ovarian cancers, meaning other hereditary cancers could be overlooked in the absence of family history documentation.
- Vagueness of Risk Categories: The broad classifications of average, moderately increased, and significantly increased risk can sometimes lack precision, causing uncertainty in clinical decision-making.
- No Consideration for Environmental Factors: Fraboc did not take into account environmental factors or lifestyle choices, which are also known to influence cancer risk, thus limiting its scope in assessing overall cancer susceptibility.
6. The Evolution of Cancer Risk Assessment Models
- From Fraboc to Modern Tools: Over time, as genetic testing advanced, newer risk assessment models began to integrate genetic information alongside family history, leading to a more precise understanding of cancer risk.
- Genetic Testing and Screening: Modern models often include genetic testing for known cancer-related mutations (such as BRCA1 and BRCA2), offering a more accurate prediction of cancer risk.
- Integration of Other Factors: Contemporary models take into account a broader range of factors, including lifestyle, environmental exposures, and personal medical history, providing a more holistic view of cancer risk.
- Personalized Medicine: Today’s risk assessment tools are more individualized, offering personalized recommendations for screenings, preventive treatments, and lifestyle changes based on genetic and environmental factors.
- Continued Relevance of Family History: Despite the advancements in genetic technology, family history remains a critical component of modern cancer risk assessments, with structured family history reviews forming the foundation for genetic testing and clinical recommendations.
7. The Future of Cancer Risk Assessment
- Advancements in Genetic Research: As our understanding of genetics continues to grow, future cancer risk assessment models will likely incorporate an even broader array of genetic mutations and biomarkers to predict risk.
- Machine Learning and AI: The use of machine learning and artificial intelligence may play a key role in analyzing large datasets of genetic, environmental, and family history information, improving the accuracy of risk assessments.
- Personalized Risk Algorithms: Future models will likely evolve to provide highly personalized cancer risk algorithms, integrating not only genetics but also lifestyle data and personal health information.
- Public Health and Education: Educating the public about the role of family history in cancer risk will continue to be a vital part of public health campaigns, especially as genetic testing becomes more accessible and widespread.
- Improved Early Detection and Prevention: The ultimate goal of modern cancer risk assessments is to improve early detection and prevention, ensuring that those at risk can receive timely interventions and reduce the overall burden of cancer.
8. Understanding the Role of Fraboc in Current Practice
- Still Part of Medical Training: While fraboc itself is no longer in active use, the principles behind it remain part of medical education, particularly in teaching how to assess family history and recognize patterns indicative of genetic risk.
- Genetic Counseling: Family history-based assessments continue to be a part of genetic counseling, where trained professionals use family history to guide decisions about genetic testing and follow-up actions.
- Clinical Application: In clinical practice, family history remains a key element in the overall risk assessment process, helping guide decisions about when to refer patients for genetic counseling or testing.
- Risk Categorization in Modern Tools: Modern tools for cancer risk assessment still use categories similar to those created by fraboc (e.g., average, moderate, high risk), but with added precision due to genetic testing and other technologies.
- Clear Communication with Patients: When terms like fraboc appear in medical records, it’s essential for healthcare providers to communicate clearly with patients about what it means and what actions, if any, need to be taken.
Conclusion
Fraboc was an important framework for assessing inherited cancer risk in the pre-genetic testing era. By focusing on family history and recognizing patterns of cancer across generations, fraboc helped clinicians make more informed decisions about cancer risk and follow-up care.
As medical knowledge advances and new technologies emerge, risk assessment models continue to evolve. However, the core idea behind fraboc—using structured frameworks to assess risk—remains a cornerstone of modern medicine. For individuals reading their medical records or considering genetic testing, understanding the history of fraboc can provide valuable context and reassurance as they navigate their cancer risk journey.